The Smarter Way to Configure, Price, and Quote Facility Services
Imagine walking into a hospital procurement meeting where the head of cardiology needs a complete diagnostic suite, the CFO demands precise pricing within budget constraints, and the compliance officer requires documentation proving every component meets FDA standards. Meanwhile, your sales rep has 24 hours to deliver a flawless quote that could make or break a million-dollar deal.
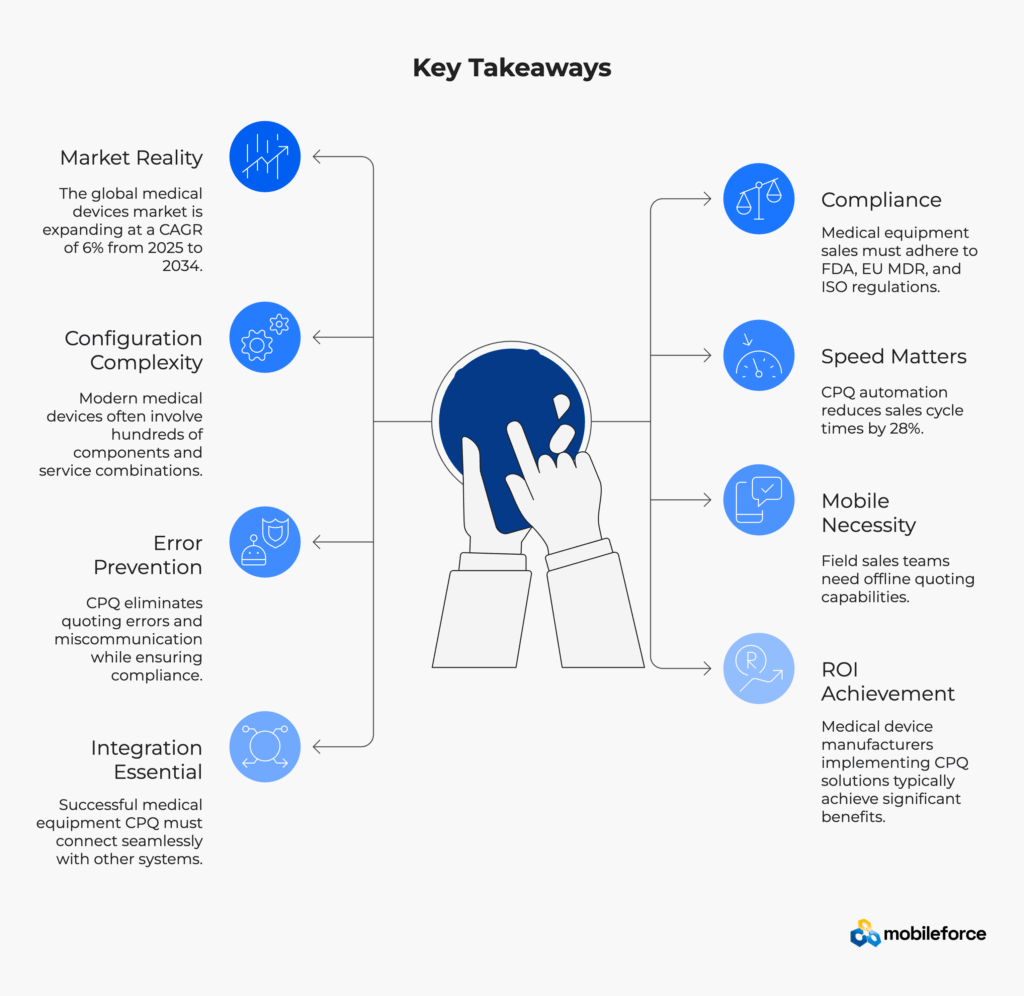
Welcome to medical equipment sales in 2025, where precision isn’t just preferred—it’s mandatory. According to the National Center for Biotechnology Information, nearly 422 million people worldwide are diagnosed with diabetes, driving unprecedented demand for medical equipment precision and compliance. Healthcare equipment procurement teams now expect sophisticated quoting capabilities, automated configuration management, and seamless integration with existing healthcare technology infrastructure.
The medical equipment industry, valued at \$678.88 billion in 2025 and expected to reach \$1,146.95 billion by 2034, faces unprecedented complexity in sales operations. Traditional quoting methods can’t handle the intricate web of product configurations, regulatory compliance, and multi-tiered pricing that define today’s medical device sales. CPQ (Configure, Price, Quote) systems specifically designed for medical equipment transform this challenge into competitive advantage, enabling sales teams to generate accurate, compliant quotes while navigating complex product portfolios and stringent regulatory requirements. Medical device sales automation through specialized CPQ platforms addresses quote-to-cash optimization, healthcare equipment configuration management, and regulatory compliance automation for medical technology companies seeking operational excellence and revenue growth acceleration.
The medical equipment industry operates in a realm where “close enough” doesn’t exist. Every component, every configuration, and every price point must align with complex regulatory frameworks while meeting precise clinical requirements. Healthcare equipment procurement specialists increasingly demand sophisticated sales tools capable of handling multi-vendor configurations, regulatory documentation automation, and real-time pricing calculations.
Medical device sales representatives face unique challenges that traditional CPQ systems cannot address: intricate product interdependencies, evolving regulatory landscapes, complex service agreement structures, and diverse stakeholder approval processes. These factors necessitate specialized configure price quote software designed specifically for medical technology companies and healthcare equipment manufacturers.
Medical devices aren’t simple products—they’re sophisticated systems. A single MRI machine might include the base unit, specialized coils, software packages, installation services, training modules, and ongoing maintenance contracts. Each component must be compatible not just technically, but also from a regulatory standpoint. The World Health Organization emphasizes that medical devices must meet stringent quality and safety standards to ensure patient safety.
Medical equipment configuration challenges include:
Medical devices are highly specialized, must adhere to strict regulations, and require precise configurations tailored to clinical needs. This complexity multiplies when considering international markets, where regulatory requirements vary significantly between regions. Healthcare technology procurement teams expect automated validation of these complex interdependencies during the quoting process.
Traditional pricing models break down in medical equipment sales. Consider a hospital purchasing a diagnostic imaging system requiring sophisticated pricing calculation methodology:
Healthcare equipment pricing complexity extends beyond simple product costs to include installation logistics, facility preparation requirements, ongoing support commitments, and regulatory compliance documentation. Medical device pricing software must accommodate these multifaceted pricing structures while maintaining accuracy and compliance across all revenue streams.
Pricing and costs can vary due to materials, regions, service/maintenance, etc., making manual calculation both time-consuming and error-prone. McKinsey research shows that US healthcare expenditure could experience 7% growth from 2022 to 2027, intensifying the need for precise pricing strategies and automated pricing calculation tools for medical equipment sales teams.
Struggling with complex medical equipment pricing scenarios? See how Mobileforce simplifies multi-dimensional pricing in a personalized demo tailored to your product portfolio.
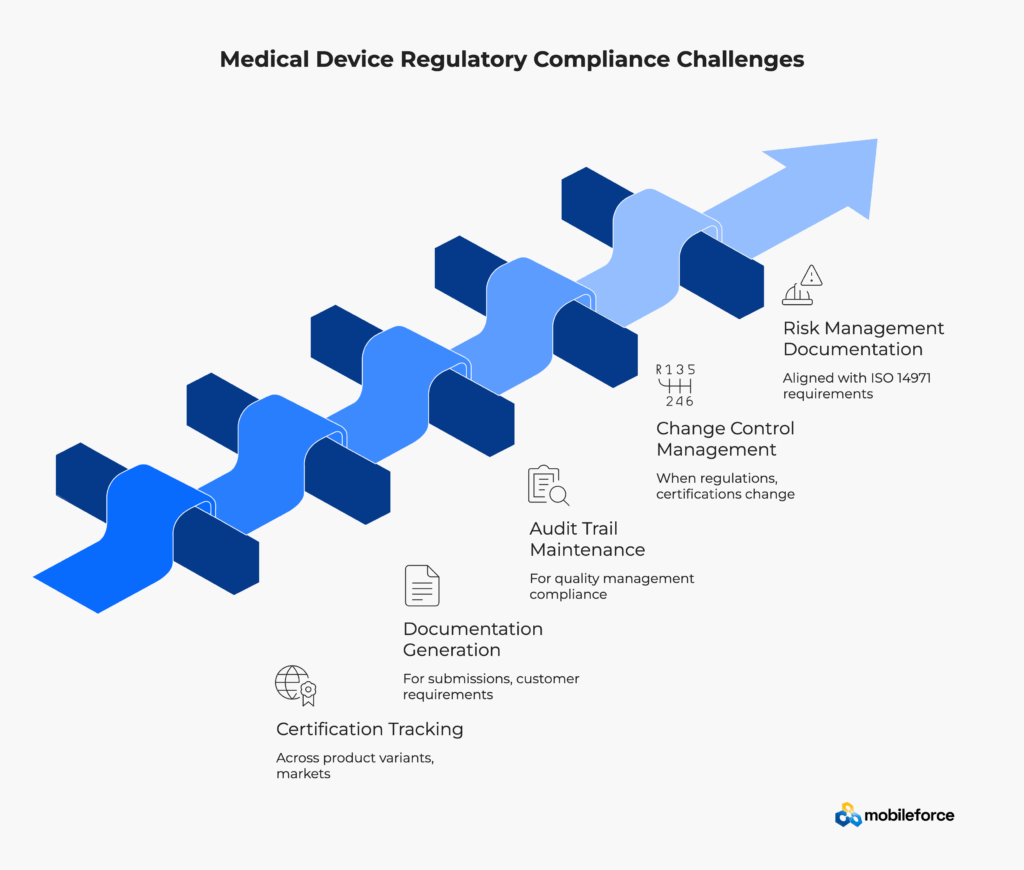
The medical device industry is highly regulated, with oversight from agencies like the FDA, European Medicines Agency, and others worldwide. Every quote must demonstrate compliance with:
Medical device regulatory compliance software must address these comprehensive requirements through automated documentation generation, audit trail maintenance, and real-time compliance verification. Healthcare equipment manufacturers face significant financial and reputational risks when quotes contain non-compliant configurations or inadequate documentation.
Regulatory compliance challenges in medical equipment sales include:
Manual quoting processes struggle to maintain the audit trails and documentation required for regulatory compliance, creating significant business risk. Healthcare technology sales teams require automated compliance verification tools that integrate regulatory requirements directly into the configuration and pricing process.
Medical equipment CPQ platforms must go beyond basic configuration and pricing to address industry-specific challenges with specialized functionality. Healthcare technology sales teams require comprehensive quote-to-cash automation, regulatory compliance management, and multi-stakeholder workflow orchestration capabilities.
Successful medical device sales automation depends on integrated CPQ functionality addressing configuration complexity, pricing optimization, compliance verification, and customer experience enhancement. Healthcare equipment manufacturers need sophisticated tools capable of handling intricate product portfolios, diverse pricing models, and stringent regulatory requirements.
Medical equipment CPQ systems embed regulatory requirements directly into the configuration process. CPQ solutions filter configuration options based on geographic regulations through automatic system filters, preventing non-compliant combinations from reaching customers.
Real-world application: When configuring a surgical laser system for European markets, the CPQ automatically excludes components that lack CE marking while suggesting compliant alternatives that meet the same clinical requirements.
According to Aberdeen Group research, CPQ automation has been shown to reduce sales-cycle times by 28%, particularly critical in competitive medical equipment markets where time-to-quote can determine deal outcomes.
Ready to see how automated compliance rules can accelerate your medical equipment sales? Discover Mobileforce’s no-code configuration capabilities with a live demonstration.
Capability | Traditional Approach | Medical Equipment CPQ |
Regulatory Compliance | Manual verification, prone to errors | Automated rule-based validation |
Configuration Options | Static catalogs, limited combinations | Dynamic, rules-driven configurations |
Documentation | Manual generation, inconsistent | Automated, audit-ready documents |
Approval Workflows | Email chains, unclear ownership | Structured, traceable approval paths |
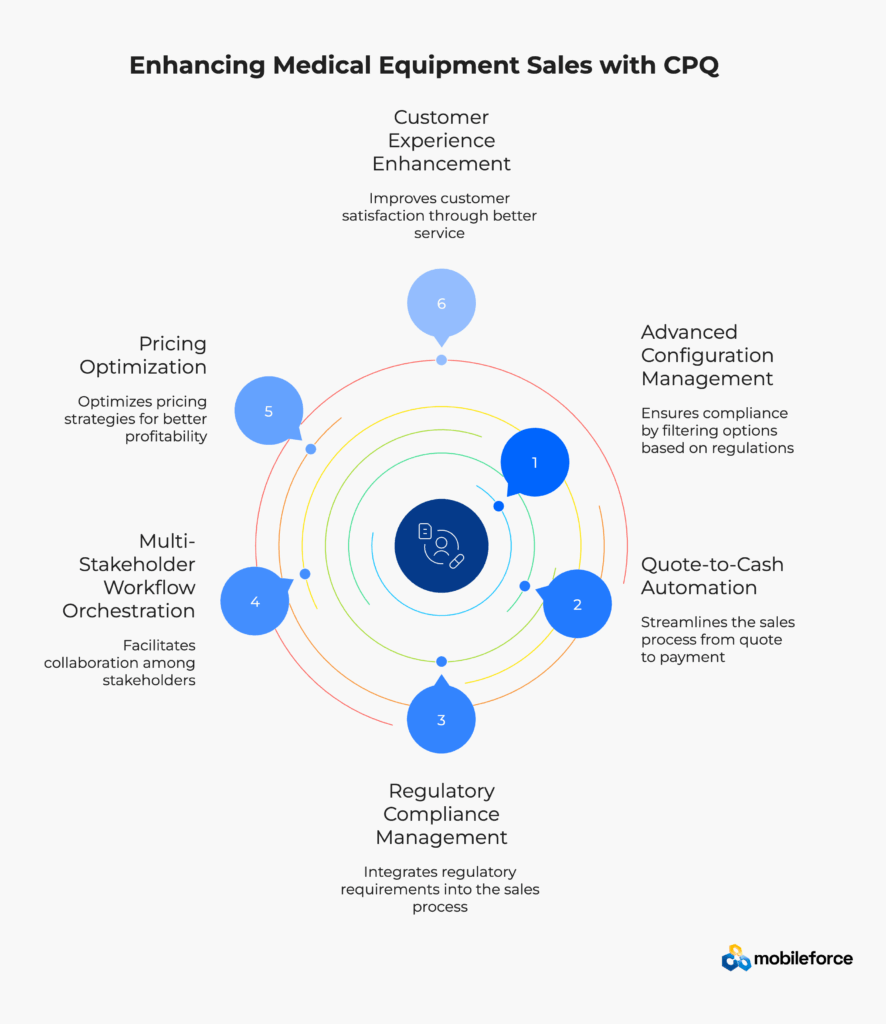
Medical equipment sales typically involve multiple revenue models within a single transaction. Leading CPQ platforms handle:
Research from Deloitte indicates that medical technology companies are increasingly shifting toward outcome-based pricing models, requiring CPQ systems capable of handling complex, multi-dimensional pricing structures. Integration with field service management systems becomes essential for medical equipment manufacturers offering comprehensive service packages alongside equipment sales.
Mobileforce’s CPQ platform excels in this area with its Revenue Engagement Cloud that connects CPQ, selling, and field service management into one seamless flow. The platform’s no-code configuration capabilities enable medical equipment manufacturers to set up complex pricing rules and product configurations without technical dependencies.
A compliant system must ensure traceability from quote to delivery. Medical equipment CPQ systems automatically generate required documentation including:
This automated approach ensures consistency while reducing the administrative burden on sales teams.
Hospital environments often have restricted network access, making mobile and offline capabilities essential. Modern enterprise CPQ solutions address this need by offering robust mobile and offline quoting capabilities. According to the U.S. Department of Health and Human Services, healthcare facilities increasingly require technology solutions that can operate in complex network environments while maintaining data security.
Advanced medical equipment CPQ platforms now incorporate AI-powered assistance to guide sales teams through complex configurations and suggest optimal pricing strategies based on historical data and market conditions.
Field sales engineers can:
Managing field sales teams across multiple hospital locations? See how Mobileforce’s mobile CPQ solution enables your sales engineers to quote complex medical equipment configurations offline and sync seamlessly.
Medical equipment manufacturers operate complex technology environments requiring seamless data flow between systems:
The National Institute of Standards and Technology (NIST) emphasizes that healthcare technology integrations must maintain robust cybersecurity protocols, particularly critical for CPQ systems handling sensitive pricing and customer data. Medical equipment manufacturers with partner programs require additional CPQ functionality to support channel sales, dealer pricing, and distributor management workflows.
Mobileforce offers seamless integration with CRM and back-office systems — 100% extensible, customizable & accurate, ensuring that quotes reflect current inventory levels and accurate costing data.
Need CPQ that integrates seamlessly with your existing medical device manufacturing systems? Explore Mobileforce’s integration capabilities with a technical demonstration of API connectivity and data synchronization.
Successfully deploying CPQ in medical equipment sales requires careful planning and attention to industry-specific requirements. Healthcare technology implementation projects demand specialized expertise in medical device regulations, complex product configurations, and healthcare procurement processes.
Medical device CPQ implementation success depends on comprehensive change management, stakeholder alignment, and deep understanding of healthcare equipment sales workflows. Leading healthcare technology consultants recommend systematic approaches addressing organizational readiness, technical requirements, and user adoption strategies.
Medical equipment sales involve multiple stakeholders, each with distinct requirements. PwC research shows that medical device procurement decisions typically involve 6-8 stakeholders, each with specific evaluation criteria:
Successful implementations begin with comprehensive stakeholder interviews to understand workflow dependencies and approval processes. Medical equipment sales often involve complex contract lifecycle management requiring integration between CPQ systems and legal document management platforms.
CPQ provides real-time data on configurations, ensuring every quote reflects exact device specs, materials, and components. This requires:
Advanced CPQ platforms incorporate automated document creation capabilities, generating regulatory compliance documentation, technical specifications, and service agreements automatically based on configured products.
The heart of medical equipment CPQ lies in intelligent configuration rules that prevent errors while enabling customization. Gartner research indicates that rule-based configuration systems can reduce product configuration errors by up to 90% in complex manufacturing environments. Effective rule sets include:
The Healthcare Information and Management Systems Society (HIMSS) reports that automated configuration rules significantly improve healthcare technology deployment success rates by ensuring proper component compatibility from the initial quote stage.
Want to see intelligent configuration rules in action for your medical equipment portfolio? Schedule a personalized Mobileforce demonstration to explore rule-based configuration for your specific product lines.
The platform’s user-friendly design ensures that even those with minimal technical expertise can utilize it effortlessly. However, successful adoption requires:
Implementation Phase | Duration | Key Activities | Success Metrics |
Discovery & Planning | 2-4 weeks | Stakeholder interviews, process mapping | Requirements documentation complete |
Configuration & Setup | 4-8 weeks | Product catalog setup, rule configuration | System configured for pilot products |
Testing & Validation | 2-4 weeks | User acceptance testing, compliance validation | All test scenarios passing |
Training & Rollout | 2-3 weeks | User training, go-live preparation | User adoption targets met |
Optimization | Ongoing | Performance monitoring, continuous improvement | KPI targets achieved |
Typical Implementation Scenario:
Based on industry implementations, a global medical device manufacturer specializing in diagnostic imaging systems would typically face challenges including manual quoting processes taking 10-14 days, high error rates, and compliance audit findings.
Implementation Approach:
Industry-Typical Results:
Based on aggregated industry data and Aberdeen Group research, medical equipment manufacturers implementing specialized CPQ solutions typically achieve:
These results represent typical industry outcomes based on published research and are not guaranteed performance metrics. Individual results may vary based on implementation scope and organizational factors.
Key Success Factors:
See how your medical equipment business could achieve similar results. Request a personalized ROI analysis and demo from Mobileforce’s medical equipment specialists.
Based on industry analysis and implementation best practices, medical equipment manufacturers should consider the following strategic approach to CPQ adoption.
Begin with a comprehensive audit of your current quoting process:
Organizations should also conduct comprehensive total cost of ownership analysis to understand the full financial impact of CPQ implementation beyond initial licensing costs.
Choose a CPQ platform that specifically addresses medical equipment challenges:
Mobileforce’s platform meets these criteria with its no-code platform that simplifies complex quoting, streamlines scheduling and dispatch, and turns CRM insights into real-time action. Organizations should evaluate CPQ pricing models carefully to ensure alignment with implementation scope and expected ROI outcomes.
Organizations transitioning from legacy quoting systems should consider professional migration services to ensure smooth data transfer and minimal business disruption during CPQ implementation.
The medical equipment industry’s complexity demands sophisticated solutions that traditional quoting methods simply cannot deliver. Configure price quote software represents more than operational efficiency—it’s a strategic enabler for sustainable growth in an increasingly competitive marketplace.
Medical device manufacturers implementing specialized CPQ solutions position themselves to capture the projected market growth to $1,146.95 billion by 2034 while maintaining the precision and compliance standards their industry demands. Advanced platforms like Mobileforce’s Field Revenue Cloud extend CPQ functionality to include field service optimization and revenue cycle management.
The question facing medical equipment sales leaders isn’t whether to invest in CPQ technology, but rather how quickly they can implement solutions that transform their competitive positioning. Every manual quote represents lost opportunity for faster response times, improved accuracy, and increased revenue capture.
Organizations seeking deeper insights into medical equipment CPQ implementation strategies can explore additional resources through Mobileforce’s comprehensive blogs covering advanced topics in revenue operations and sales automation for healthcare manufacturers.
Ready to join the leaders in medical equipment sales automation? Schedule your comprehensive Mobileforce demonstration and discover how specialized CPQ can transform your complex pricing and compliance requirements while accelerating your sales cycles in today’s demanding medical equipment market.
Medical equipment quoting involves complex regulatory compliance requirements, multi-component configurations, and service-intensive sales models that standard CPQ systems aren’t designed to handle. Medical devices and equipment manufacturing operates in one of the most complex and highly regulated environments in the world, requiring specialized functionality for compliance management and audit trails. Healthcare equipment sales teams must navigate FDA regulations, ISO certifications, and regional compliance requirements while managing sophisticated product configurations and pricing structures.
Yes, modern medical equipment CPQ platforms support multiple revenue models within a single quote, including hardware sales, software subscriptions, service contracts, and consumables pricing. This capability is essential for medical equipment sales where customers often purchase integrated solutions combining products and services. Healthcare technology CPQ systems must accommodate subscription billing, usage-based pricing, maintenance contracts, and training services within unified quotations.
CPQ systems use rule-based configurations for products, which automatically enforce compliance. If a combination of product options has not been approved by regulatory bodies, the system will mark it invalid based on the configuration rules. This prevents non-compliant products from being quoted while maintaining complete audit trails for regulatory reporting. Medical device compliance software integrated with CPQ platforms ensures all quotes meet FDA, EU MDR, and ISO requirements automatically.
Medical device company Nevro saw a 178% ROI and had their payback period in less than eight months after adding CPQ. ROI factors include reduced quote processing time, improved accuracy, faster sales cycles, and decreased administrative costs. Healthcare equipment manufacturers typically achieve 60-80% reduction in quote turnaround time and 15-25% revenue increases through improved win rates and pricing optimization.
Medical equipment CPQ platforms provide offline functionality that allows sales teams to configure products and generate quotes without internet access. Once connectivity is restored, the system automatically synchronizes all data, ensuring quotes and customer information remain current across the organization. Healthcare facility sales often require mobile CPQ capabilities due to network restrictions and security protocols in hospital environments.
Yes, leading CPQ platforms offer extensive integration capabilities with CRM systems like Salesforce, ERP systems like SAP, and specialized medical device manufacturing software. Mobileforce provides seamless integration with CRM and back-office systems — 100% extensible, customizable & accurate. Healthcare technology integration requirements include real-time inventory data, pricing synchronization, and order management connectivity.
Training requirements vary by role, but most platforms are designed for ease of use. Cincom CPQ’s intuitive interface enables sales teams to configure products quickly and generate accurate quotes instantly. Successful implementations typically include role-specific training programs, hands-on workshops, and ongoing support resources. Medical device sales training should cover regulatory compliance, product configuration rules, and pricing methodology.
Advanced CPQ platforms automatically adjust quotes based on target markets, switching regulatory requirements, currencies, languages, and compliance documentation as needed. CPQ platforms handle global products and compliance-related requirements easily and effectively. International medical equipment sales require automated regulatory mapping, certification tracking, and documentation generation for different geographic markets and healthcare systems.